
Philadelphia, PA: Wolters Kluwer Health 2012.\ m\). 1 Interaction Processes 1.1 Photoelectric Effect 1.2 Compton Effect 1.3 Coherent Scatter 2 Radiation Biology 2.1 Biomolecular Damage 2.2 Tissue Damage 2.3 Health Risks 3 Tissue Attenuation 3.1 Soft Tissue 3.2 Bone 3.3 K-Edge Effect 4 X-Ray Shadows 4.1 Imaging Geometry 4.2 Image Distortion 5 Subject Contrast 5. Reprinted with permission from Fosbinder RA, Orth D. Compton effect definition: a phenomenon in which a collision between a photon and a particle results in an increase. The intensity of the scattered xray is measured as a function of the wavelength shift, where (2.4. Scattering means dispersing in different directions, and inelastic means that energy is lost by the scattered object in the process. As the atomic number of the absorber decreases, the probability of characteristic X-ray emission decreases. The Compton effect concerns the inelastic scattering of xrays by electrons. The difference in binding energy is released as either characteristic X-rays or Auger electrons. This will, in turn, create another vacancy, which is filled from an even lower binding energy shell, creating an electron cascade from outer to inner shells. An electron from a more outer shell, with a lower binding energy, will fill this vacancy. As a result of a photoelectric interaction, atoms are ionized with a vacancy of an inner shell electron. For example, photons whose energies exceed the K-shell binding energies will most likely result in photoelectric interactions with the K-shell electrons. The energy which is lost by this electron as it drops to the inner shell is emitted as characteristic radiation (an x-ray photon) or as an Auger.
To stabilize the atom an outer shell electron fills the vacancy in the inner shell. For a photoelectric effect to occur, the incident photon energy must be equal to or greater than the binding energy of the ejected electron. Hence, the photoelectric effect contributes to the attenuation of the x-ray beam as it passes through matter.
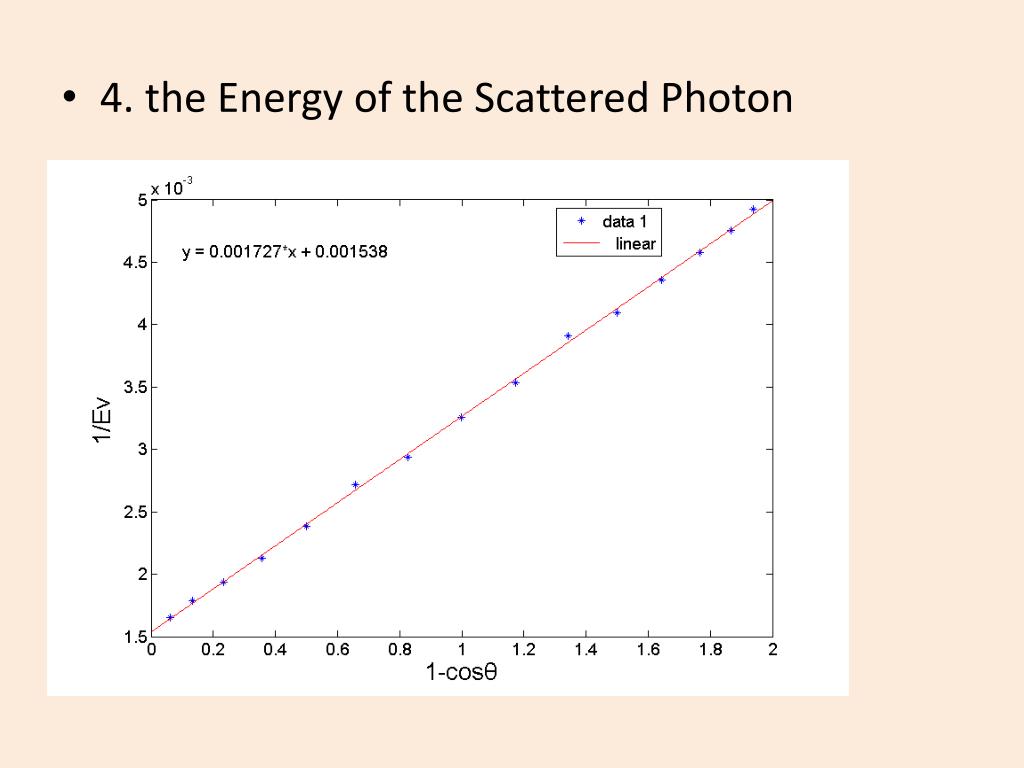
#GRAPH COMPTON EFFECT VERIFICATION#
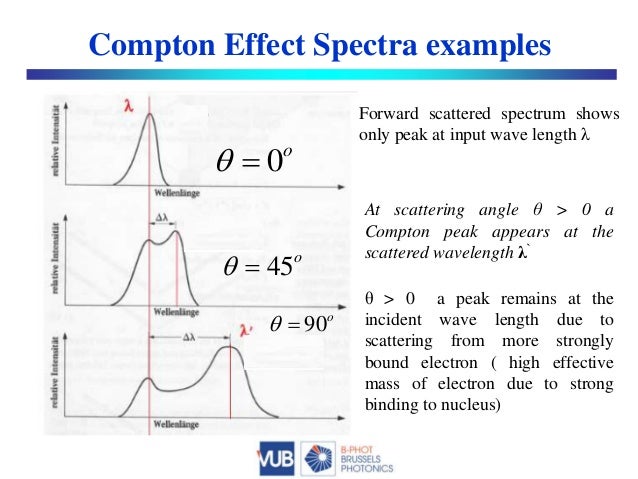
Also, the probability of photoelectric absorption generally decreases with the increase in photon energy. EXPERIMENTAL VERIFICATION OF COMPTON EFFECT Principle: When a photon of energy h collides with a scattering element, the scattered beam has two components, viz., one of the same frequencies or wavelength as that of the incident radiation and the other has lower frequency or higher wavelength compared to incident frequency or wavelength.
This is why barium ( Z = 56) and iodine ( Z = 53), which have high atomic numbers, are used as contrast agents in X-ray imaging. Therefore, as the atomic number increases, the photoelectric absorption effect becomes more pronounced. The probability of photoelectric absorption, by approximation, is considered proportional to Z 3/E 3, where Z is the atomic number and E is the energy of the incident photon.


 0 kommentar(er)
0 kommentar(er)
